Research-Grade Products & Services
I Peace’s Research Services
From a single vial of cells to drug discovery, I Peace offers a comprehensive set of research products and services to support your R&D needs.
Research Grade iPSC Products & Services
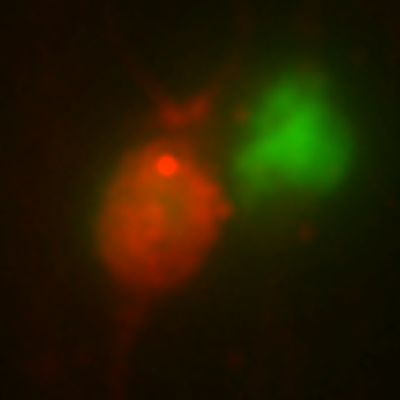
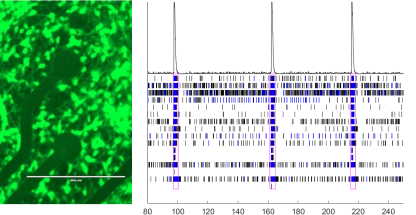
As iPSC experts, I Peace offers everything you’ll need for your iPSC journey. With a comprehensive offering of off-the-shelf lines and custom line development and manufacturing services, I Peace is sure to be your best and most reliable iPSC partner. Researchers from every level of experience will benefit from our short turnaround time (2 months from induction to delivery), mass induction capabilities (more than 100 clones at a time), all with no residual factors and in-depth cell characterization, including genomic analysis and differentiation potential. Combine our iPSC research products and services with our disease modeling services to fast-track your drug screening research.
-
Short lead time
Two month from induction to delivery -
Expansion
100 clones at a time

Off-the-Shelf iPSC Lines
To advance all aspects of the research and development of new therapies, I Peace offers a variety of human iPSC lines with robust differentiation capacity such as neuron, hepatocyte, MSC, immune cells and cardiomyocyte. They are fully characterized under QC. For companies working on pre-clinical research, GMP-derived iPSC lines create a smooth transition from research to clinical trial, while companies working on T-cell therapies will benefit from our alpha beta and gamma delta T cell derived iPSCs with confirmed capacity to differentiate back to rejuvenated, functional T cells. I Peace also offers a cord blood derived iPSC line designed for culturing in 3D.
GMP-derived research-grade iPSC lines
- CNTB Series iPSC – Set of five lines
- MUPI Series iPSC – Set of two lines
Pure Research-grade iPSC lines
- Alpha Beta T derived iPSC – 3 lines
- Gamma Delta T derived iPSC – 2 lines
- K5 (3D cell culture)
iPS Cell Services
iIn addition to our off-the-shelf lines, I Peace offers end-to-end custom iPS cell generation. Whether you need everything from donor sourcing to terminally differentiated cells, or any part in between, I Peace’s team of cell and manufacturing experts’ tailors services to meet your research goals.
- Donor sourcing
- iPSC induction
- iPSC expansion (seed banks, master cell banks, working cell banks)
- iPSC differentiation
- iPSC and iPSC- derived cell characterization
iPSC-Derived & Primary Cell Products & Services
iPSC-derived cells solve the scale-up difficulties and lot-to-lot variability associated with primary human cells. I Peace has extensive experience differentiating iPSCs into multiple cell types. I Peace offers tailored differentiation services, using our cells or yours, to generate cell types for your research studies.
Available iPSC Differentiation Services:
- iPSC-derived neurons & NPCs
- iPSC-derived NK cells
- iPSC-derived αβT cells
- iPSC-derived γδT cells
- iPSC-derived HSCs
- iPSC-derived MSCs
- iPSC-derived hepatocytes
- iPSC-derived cardiomyocytes
Primary Cell Products & Expansion Services
I Peace’s experienced research team has developed proprietary methods to expand primary cells, including NK, αβ, and γδ T cells, while preserving cell function, overcoming previous limitations of culturing primary immune cells. Our NK and γδT cells are also available for purchase to support your research needs.
Products
- αβ T cells
- NKT cells
- γδT cells
Services
- NKT cell expansion
- γδT cell expansion
- αβT cell expansion
Disease Modeling
Drug Screening and Discovery Services
Harness the power of iPS cells by utilizing their unique ability recapitulate diseases in vitro overcoming the challenges of donor sourcing and potentially invasive tissue sampling and enabling scientists to study the biological mechanisms of even the rarest human diseases. At I Peace, we offer a suite of iPSC-based disease modeling services to facilitate the understanding of human disease mechanisms and accelerating drug discovery and screening for a happier, healthier future.
Read on to learn about our disease modeling services as well as some of our previous disease models used to support drug screening efforts.
I Peace iPSC Disease Modeling Services
- Establishment of patient iPS & iPS-derived cell lines
- Disease modeling
- Drug screening
- Drug discovery
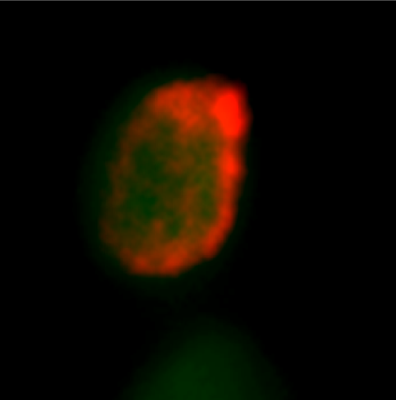
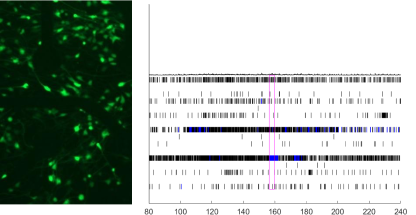
Comparison study of healthy donor and neurological disease patient:
iPSC-derived neurons were created to measure action potentials and synchronization of action potentials on MicroElectrode Array (MEA) plates. The results showed that synaptogenesis was predominantly suppressed in patients with neurological diseases and no synchronized action potential entrainment was observed.
Healthy Person
Wild Type iPSCs
Wild Type
Neural Cells
Patient
Patient-derived iPSCs
Patient-derived
Neural Cells
"Puel" iPSC Cell Culture Medium
Our “Puel” human iPSC culture medium provides researchers with a consistent homogeneous and undifferentiated phenotype in 2D or 3D, with or without feeder cells. The cultured iPSC lines in Puel have a good differentiation potential. GMP-grade coming soon!

Key Features
- Developed by iPSC experts
- Competitive pricing
- High cell survival rate and genomic stability
- Compatible for low-density iPSC culture
- Suitable for 2D/3D, feeder-free/feeder cultures
- GMP-compatible composition for a smooth transition from research to clinical manufacturing
- GMP-grade version and FDA DMF coming soon