What are iPSCs?
What are iPSCs?
What are iPSCs?
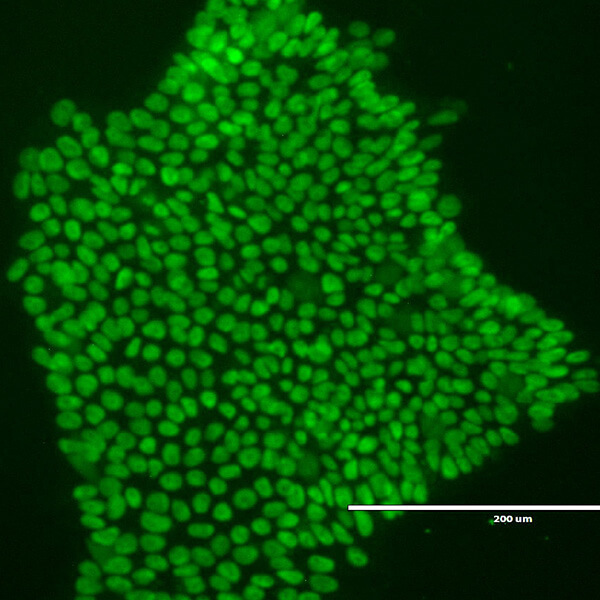
iPSC stands for induced pluripotent stem cells and were originally developed by Professor Shinya Yamanaka who received the 2012 Nobel Prize in Physiology or Medicine for his work. iPSCs are generated from adult somatic cells such as skin or blood cells by introducing reprogramming factors (genes) using vectors such as plasmids, RNA or viral vectors. iPSCs can be differentiated into various cells of the body such as neurons, heart cells, cartilage, blood cells, T cells, liver cells, and β cells that produce insulin.
Because appropriate amounts of the necessary cell type can be generated from one’s own cells and transplanted, iPSCs are an ideal platform for cell therapy and regenerative medicine. Clinical trials have started all over the world, including in Japan and the United States. Additionally, iPSC technology enables production of specific somatic cells from patients with various diseases. These somatic cells can then be used for drug screening, toxicity testing, and diagnostics. Researchers have also used iPSC-derived somatic cells to investigate disease mechanisms and as a result, various effective drug candidates have been discovered or re-discovered. iPSCs are indispensable for shaping the future of medical care.
Main features of iPSC
There are three main features that make iPSCs ideal for research, clinical and diagnostic applications.
1.Ability to be produced from anyone’s cells.
iPSCs can be made by introducing special factors into various types of cells such as blood, skin, and hair cells and culturing them. With the current technology, iPSCs can be made with just 5 mL of blood.
1.Ability to be produced from anyone’s cells.
iPSCs are made by introducing four special factors into various types of cells such as blood, skin, and hair cells, and culturing them. With current technology, iPSCs can be made with just 5 mL of blood.
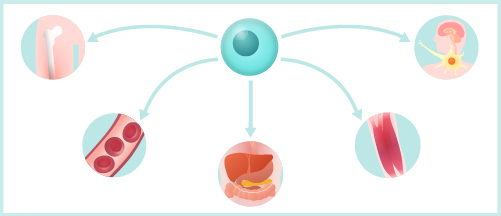
2. Ability to be transformed into cells of various tissues and organs.
2. Ability to be transformed into cells of various tissues and organs.
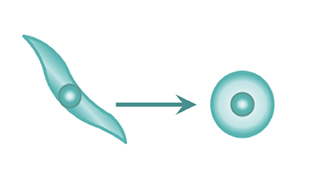
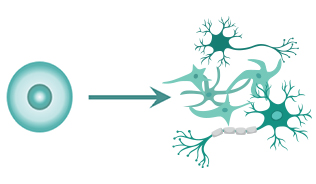
A major feature of iPSCs is that they have pluripotency. Pluripotency denotes the capability of the cells to become differentiated into cells of various types, such as neurons or cardiomyocytes.
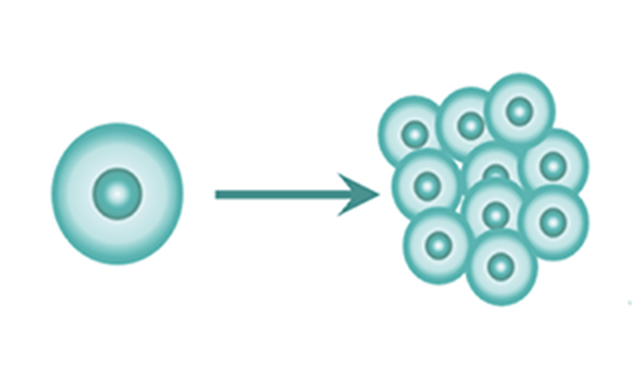
3. Ability to proliferate almost indefinitely.
3. Ability to proliferate almost indefinitely.
Since iPSCs can divide and proliferate well, they can be cultured and expanded with ease.
Application of iPS cell technology
1. Application for regenerative medicine
2.Drug development, investigating disease mechanism
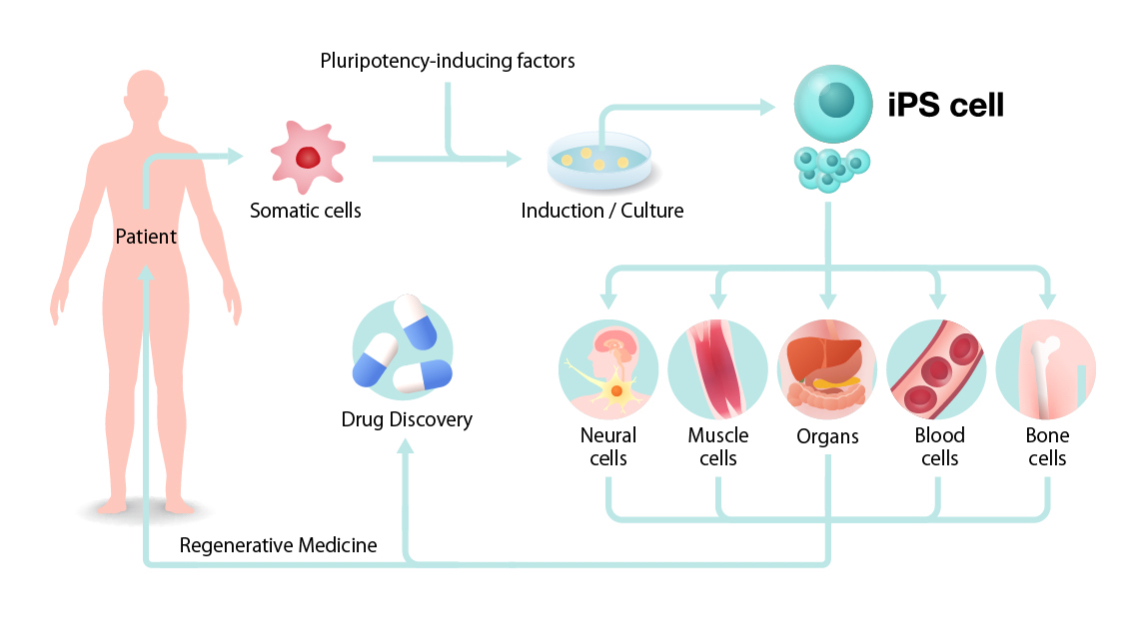
How will iPSCs be used for regenerative medicine, drug discovery, and elucidation of pathological mechanisms? First, iPSCs are prepared by collecting cells from the patient’s blood and skin and introducing reprogramming genes. Since large numbers of cells are required for cell transplantation and drug discovery, once iPSCs are generated, they are expanded (to increase the number of cells). By transforming a large number of iPSCs to target cells such as neurons, cardiomyocytes, blood cells, bone, etc., it is possible to obtain enough cells for cell transplantation treatment and drug discovery. The resulting cells can be roughly divided into two uses.
One is regenerative medicine that improves the functions of the body by transplanting cells such as the differentiated cardiomyocytes and neurons into the human body. Since Professor Shinya Yamanaka announced the successful generation of human iPSCs in 2007, various studies have been conducted for clinical applications in Japan and overseas. Examples of diseases for which clinical research has already begun include age-related macular degeneration, Parkinson’s disease, severe ischemic cardiomyopathy, and spinal cord injury. In addition, research is being conducted on application to diabetes, corneal disease, cerebral infarction, and liver and kidney diseases, among many others.
Also, CAR-T cell therapy research is attracting attention as one example of cancer treatment using iPSCs. This is an immunotherapy in which a patient’s blood-derived iPSCs are genetically modified to produce T cells that can recognize and attack specific cancer cells when returned to the patient’s body. The second application is to reproduce disease in a culture dish using iPSCs and use it to study disease mechanisms and develop new drugs. Utilizing iPSCs for drug discovery has two significant benefits. One is that it can be performed using human cells, as opposed to animals. Second, iPSCs can grow indefinitely, so researchers can get as many cells as needed. By successfully combining cells made from patient-derived iPS cells with animal experiments, we can efficiently develop new drugs in a short period of time and evaluate drug efficacy more accurately. In addition, the effects and side effects of individual drugs may differ due to differences in genetic background and environmental factors. With iPSC technology, optimal drug for each individual can be tested on patient-derived iPSC-based assays so personalized medicine is no longer a dream. In particular, iPSCs can greatly reduce the cost of orphan drug research and development.
How will iPSCs be used for regenerative medicine, drug discovery, and elucidation of pathological mechanisms?
First, iPSCs are prepared by collecting cells from the patient’s blood and skin and introducing reprogramming genes. Since large numbers of cells are required for cell transplantation and drug discovery, once iPSCs are generated, they are expanded (to increase the number of cells). By transforming a large number of iPSCs to target cells such as neurons, cardiomyocytes, blood cells, bone, etc., it is possible to obtain enough cells for cell transplantation treatment and drug discovery. The resulting cells can be roughly divided into two uses. One is regenerative medicine that improves the functions of the body by transplanting cells such as the differentiated cardiomyocytes and neurons into the human body. Since Professor Shinya Yamanaka announced the successful generation of human iPSCs in 2007, various studies have been conducted for clinical applications in Japan and overseas. Examples of diseases for which clinical research has already begun include age-related macular degeneration, Parkinson’s disease, severe ischemic cardiomyopathy, and spinal cord injury. In addition, research is being conducted on application to diabetes, corneal disease, cerebral infarction, and liver and kidney diseases, among many others.
Also, CAR-T cell therapy research is attracting attention as one example of cancer treatment using iPSCs. This is an immunotherapy in which a patient’s blood-derived iPSCs are genetically modified to produce T cells that can recognize and attack specific cancer cells when returned to the patient’s body. The second application is to reproduce disease in a culture dish using iPSCs and use it to study disease mechanisms and develop new drugs. Utilizing iPSCs for drug discovery has two significant benefits. One is that it can be performed using human cells, as opposed to animals. Second, iPSCs can grow indefinitely, so researchers can get as many cells as needed. By successfully combining cells made from patient-derived iPS cells with animal experiments, we can efficiently develop new drugs in a short period of time and evaluate drug efficacy more accurately. In addition, the effects and side effects of individual drugs may differ due to differences in genetic background and environmental factors. With iPSC technology, optimal drug for each individual can be tested on patient-derived iPSC-based assays so personalized medicine is no longer a dream. In particular, iPSCs can greatly reduce the cost of orphan drug research and development.
History of iPSC
1958
Successful reprogramming of frog somatic cells
(Sir John Bertrand Gurdon)
1998
Establishment of human ES cells
2006
Establishment of mouse iPS Cells
2007
Establishment of human iPS Cells
2012
Sir John Bertrand Gurdon / Dr. Shinya Yamanaka
The Nobel Prize in Physiology or Medicine
Regenerative Medicine
2009
Human stem cell
First clinical trial approval
(ES Cell・Spinal injury)
2013
Jul AMD Clinical trial (world’s first with iPSC technology)
2018
Sep Platelet Clinical trial
Nov Parkinson disease 1st Autotransplantation
2019
Feb Spinal cord injury Clinical trial
Apr AMD 5 transplantations completed one-year follow-up
Aug Corneal disease 1st Transplantation
Dec NK Cell Cancer 1st Transplantation
2020
Jan Cartilage Clinical trial
Jan Heart disease 1st Transplantation
Drug Discovery
2016
Jan Spinal muscular atrophy Clinical trial
2017
Aug Progressive ossifying fibrodysplasia clinical trial
2016
Apr Pendred Syndrome clinical trial
Dec ALS clinical trial(total 3)
1958
Successful reprogramming of frog somatic cells
(Sir John Bertrand Gurdon)
1998
Establishment of human ES cells
2006
Establishment of mouse iPS Cells
2007
Establishment of human iPS Cells
2009
Human stem cell
First clinical trial approval
(ES Cell・Spinal injury)
2012
Sir John Bertrand Gurdon / Dr. Shinya Yamanaka
The Nobel Prize in Physiology or Medicine
2018
2013
2016
2017
2019
Feb Spinal cord injury Clinical trial
Apr AMD 5 transplantations completed one-year follow-up
Aug Corneal disease 1st Transplantation
Dec NK Cell Cancer 1st Transplantation
2020
Jan Cartilage Clinical trial
Jan Heart disease 1st Transplantation
2018
Apr Pendred Syndrome clinical trial
Dec ALS clinical trial(total 3)