Clinical-grade iPSC production and
banking service My PeaceTM
My Peace is a service that offers creating and storing clinical-grade iPS cells generated from client’s blood for use in future regenerative medicine, rejuvenating treatment and comprehensive medical exam.
For more information visit My Peace Website.
https://mypeace-personal-ipscbanking.com/en/

My PeaceTM is your own iPSCs
- My Peace is a service that creates and stores iPSCs generated from clients’ blood for use in future regenerative medicine
- We manufacture clinical-grade cells compatible with clinical use
Features of MiPSCTM, your own personal cGMP(clinical-grade) iPSCs
Advanced process management suitable for cell transplantation
I Peace manufactures and provides cGMP(clinical-grade) iPSCs to the world’s leading pharmaceutical companies. The experience, technology, and advanced quality control system also applied to the production of personal use iPSCs (My Peace). Information about each individual’s cells is recorded and stored at all stages of the manufacturing process (blood sampling → virus testing → manufacturing → storage). In order to use cells clinically, traceability and documentation of the quality of the cells themselves and how they were manufactured are necessary. At I Peace, excellence is our standard in everything we do, including My Peace.
Ability to use the cells anywhere in the world
The I Peace production facility has been approved by the Kinki Regional Bureau of Health and Welfare of the Ministry of Health, Labor and Welfare for permission to manufacture specific cell products. This is approved by on-site inspection of the manufacturing process and its control. It has also been inspected under US FDA guidelines and is certified to comply with FDA guidelines.
In addition, I Peace is actively collaborating with pharmaceutical companies, medical institutions, and regenerative medicine technology development companies around the world to accelerate utility of banked cells. We are strongly promoting development globally to make it truly accessible.
Experienced Production Staff
Our CEO Koji Tanabe is the second author of the paper that announced successful production of human iPSCs for the first time in the world. Under Professor Shinya Yamanaka of the Center for iPS Cell Research and Application, Kyoto University, he has been engaged in iPSC development from the beginning. After obtaining his doctorate, he worked on various research toward the practical application of iPSCs at Stanford University in the United States. In addition, all of our iPSC manufacturing staff have been engaged in the production of clinical-grade iPSCs for a long time, and we are proud of our deep knowledge and experience. The iPSCs created by our staff meet the highest standards of quality and safety, so you can use them with confidence.
Safe and secure cell collection system
I Peace produces iPSCs from blood cells, the most established source of iPSCs. Blood collection is performed by a safe process, similar to a blood donation, and is supervised by the former director of Kyoto University Hospital. Blood is collected directly in the blood collection tube and does not come into contact with the outside air, without contamination from bacteria and viruses floating in the air. Blood collected in this manner is well-documented to be safe for clinical use. Additionally, we test the manufactured iPSCs with stringent sterility and virus tests, and store the cells only after they pass the safety tests.
Bank your cells and become a My Peacer
MiPSCer’s cells could
- Be used for cell therapy and transplantation for your future self
- Be used for cutting-edge regenerative medicine and drug discovery research
- Save people with the same illness or genetically similar to you
- Contribute to the development of regenerative medicine
I Peace aims to make cell therapy accessible to a wide range of people through MiPSC.
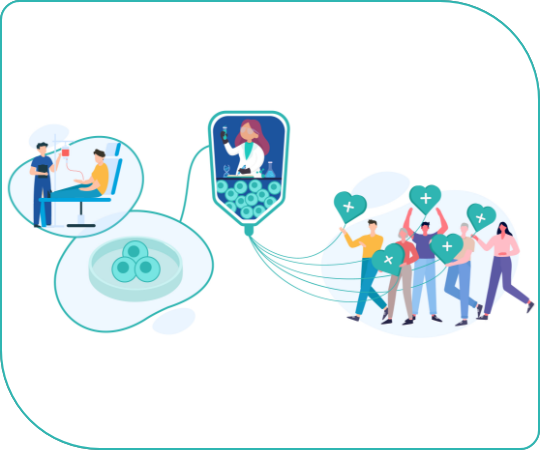
My Peacer’s cells could
- Be used for cell therapy and transplantation for your future self
- Be used for cutting-edge regenerative medicine and drug discovery research
- Save people with the same illness or those who are genetically similar to you
- Contribute to the development of regenerative medicine
The greater the number of My Peacers, the greater the collective power to meaningfully impact society. For example, we can establish a large library for drug discovery research that reflects our diversity and accelerates precision medicine development. With consent to use for transplantation in others (without compromising your personal stock), it will be easier for patients to find iPSCs that are immunologically compatible. My Peacers can actively contribute to advancing research for diseases that currently have no cures.
I Peace aims to deliver cell transplantation to a wide range of people through the My Peace service, and together with other My Peacers, we will create a future where regenerative medicine can save many people. Together, we can realize a future that will save ourselves and everyone else.



Learn more about MiPSCTM
What are the uses?
01
Use in regenerative medicine
再生医療への活用と免疫拒絶反応のリスク
再生医療とは、怪我や病気で体が失ってしまった機能を回復させるための治療です。
例えば、心臓疾患の患者さんに心筋細胞シートを移植して心臓の機能を改善させたりする治療があります。
iPS細胞を用いた細胞移植医療(再生医療)の理想形は自家移植です。つまり患者さん本人由来のiPS細胞から作製した細胞を移植して治療することです。なぜなら、他人由来のiPS細胞を利用した細胞移植には免疫拒絶反応がつきものです。HLAホモ接合体を有する方の細胞から作製したiPS細胞は、免疫拒絶反応が起きにくいと言われているものの全く免疫拒絶反応が起こらないということではありません。自分自身のiPS細胞から作製した細胞ほど自分の体にマッチする細胞はないと言えます。
02
Minimal risk of
immune rejection
再生医療への活用と免疫拒絶反応のリスク
再生医療とは、怪我や病気で体が失ってしまった機能を回復させるための治療です。
例えば、心臓疾患の患者さんに心筋細胞シートを移植して心臓の機能を改善させたりする治療があります。
iPS細胞を用いた細胞移植医療(再生医療)の理想形は自家移植です。つまり患者さん本人由来のiPS細胞から作製した細胞を移植して治療することです。なぜなら、他人由来のiPS細胞を利用した細胞移植には免疫拒絶反応がつきものです。HLAホモ接合体を有する方の細胞から作製したiPS細胞は、免疫拒絶反応が起きにくいと言われているものの全く免疫拒絶反応が起こらないということではありません。自分自身のiPS細胞から作製した細胞ほど自分の体にマッチする細胞はないと言えます。
03
Drug screening
for precision
medicine
再生医療への活用と免疫拒絶反応のリスク
再生医療とは、怪我や病気で体が失ってしまった機能を回復させるための治療です。
例えば、心臓疾患の患者さんに心筋細胞シートを移植して心臓の機能を改善させたりする治療があります。
iPS細胞を用いた細胞移植医療(再生医療)の理想形は自家移植です。つまり患者さん本人由来のiPS細胞から作製した細胞を移植して治療することです。なぜなら、他人由来のiPS細胞を利用した細胞移植には免疫拒絶反応がつきものです。HLAホモ接合体を有する方の細胞から作製したiPS細胞は、免疫拒絶反応が起きにくいと言われているものの全く免疫拒絶反応が起こらないということではありません。自分自身のiPS細胞から作製した細胞ほど自分の体にマッチする細胞はないと言えます。
04
医療を通して
社会貢献
再生医療への活用と免疫拒絶反応のリスク
再生医療とは、怪我や病気で体が失ってしまった機能を回復させるための治療です。
例えば、心臓疾患の患者さんに心筋細胞シートを移植して心臓の機能を改善させたりする治療があります。
iPS細胞を用いた細胞移植医療(再生医療)の理想形は自家移植です。つまり患者さん本人由来のiPS細胞から作製した細胞を移植して治療することです。なぜなら、他人由来のiPS細胞を利用した細胞移植には免疫拒絶反応がつきものです。HLAホモ接合体を有する方の細胞から作製したiPS細胞は、免疫拒絶反応が起きにくいと言われているものの全く免疫拒絶反応が起こらないということではありません。自分自身のiPS細胞から作製した細胞ほど自分の体にマッチする細胞はないと言えます。
01
Cell therapy in the future
02
Accelerate medical research and drug discovery
03
Protect your loved ones
04
Help patients
Discovery of new, better drugs and therapies is limited by the fact that access to patients and patients’ cell types is limited—severely, in the case of some rare diseases. iPSCs have the potential to be turned into any type of cell in the body. With consent to use for research, banked iPSCs could be distributed to research institutions and used to model diseases in a dish to help accelerate a cure. As more people’s iPSCs are generated and banked, the greater the potential for a cure.




What are the benefits of iPSCs?
Regenerative Medicine
While the cells may not be used right now, clinical research is underway for many applications
Drug screening
Help accelerate research and potentially reap the benefits in the future
Avoid immune rejection
Generate the type of cells you need if you get sick in the future
Preserve for a long time
Contribute to society by consenting use of your cells for research
Why Now?
Significance of storing your iPSCs Early
Store younger cells
Time required for
manufacturing and
quality evaluation
It takes time to manufacture iPSCs. To ensure that the manufactured iPSCs meet clinical standards, the cells need to be rigorously evaluated for quality—which takes time. We at I Peace have successfully shortened production times with a number of innovations, but it still takes time from when the order is received to when the generated cells are banked.
Prepare for an emergency
Timely treatment is critical for some injuries and illnesses. Considering that the manufacturing and quality evaluation of iPSCs takes time, if the manufacturing process begins after injury or diagnosis, it is possible that a patient misses the critical time window for a successful transplantation. If your own iPSCs have been generated, evaluated, and stored in advance, you will be able to obtain the required types of cells in the shortest possible time and use them for treatment.
Process and Price
Service Flow




MiPSCer testimonies
It’s so easy with MiPSC to make and bank cells. I’m very happy to be able to contribute to research this easily
MiPSCer A
MiPSC makes it easy to be the first in line to receive cutting edge cell therapy
MiPSCer B
Reach out to us to learn more